Our research topics
The ability to design and synthesize new molecules, and to explore their molecular architectures, is a tool of enormous power that has facilitated the development of many areas within the field of molecular science. Research in the Kirschning group aims to strategically combine a diverse range of these synthetic approaches, including biotechnological methods based on enzymes and whole cells; enabling technologies such as flow chemistry and inductive heating in organic synthesis; and the total synthesis of complex organic molecules, to initiate biomedical studies of new molecules and materials with promising therapeutic potential.
BioMed: Biomedical materials
Polysaccharide-based hydrogels – a versatile toolbox for tissue engineering
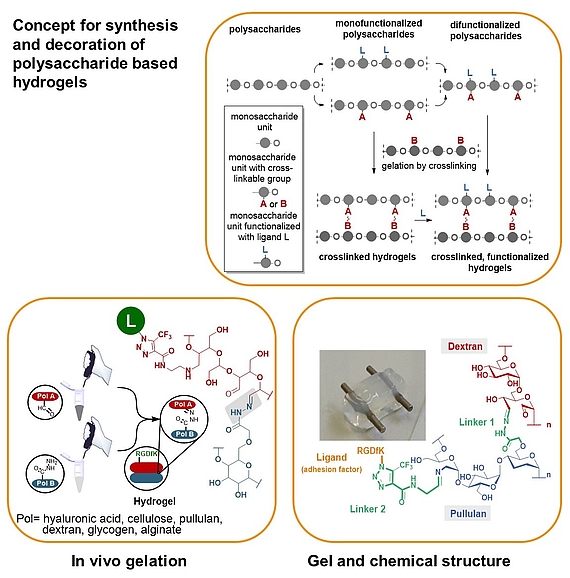
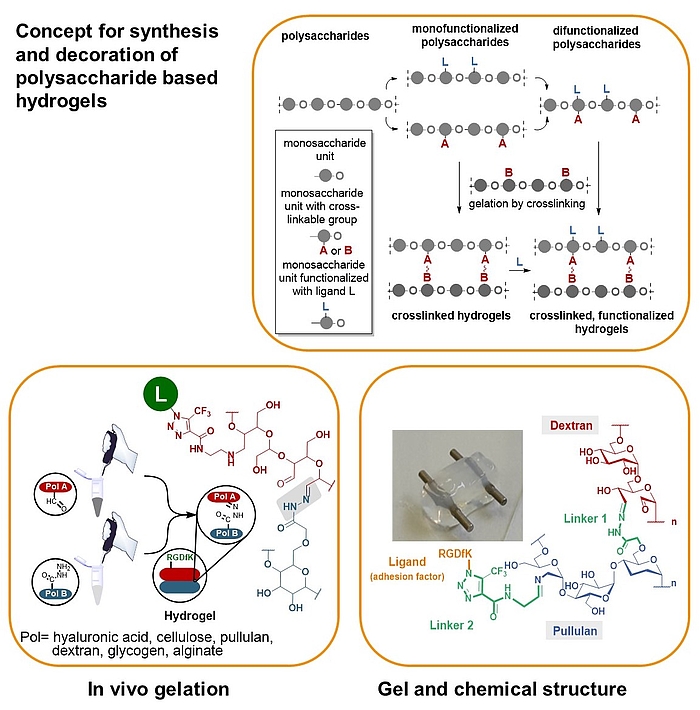
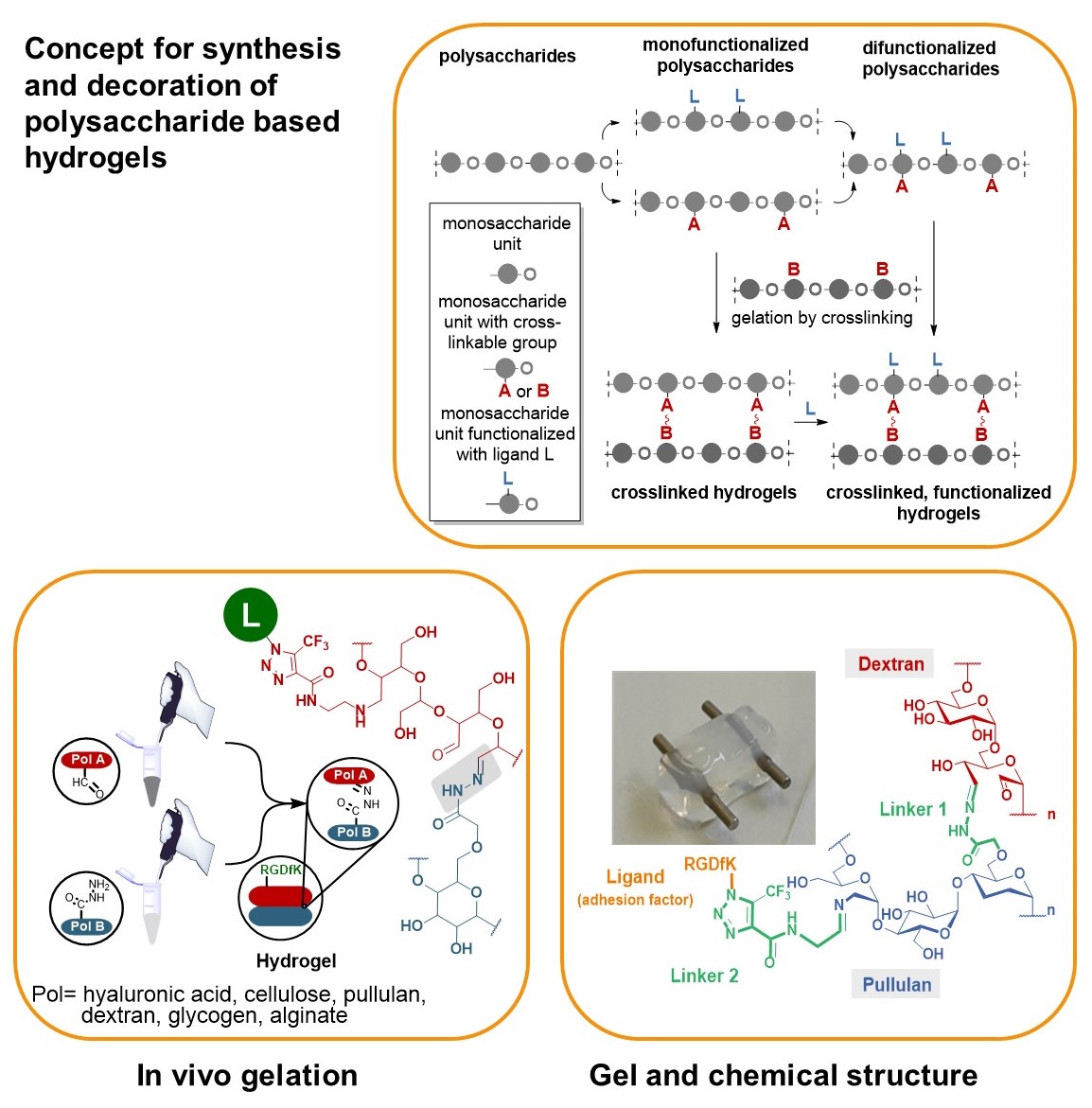
We developed a synthetic tool box for functionalizing polysaccharides (hyaluronic acid, alginate, dextran, pullulan, glycogen, lentinan and carboxymethyl cellulose) with “clickable” aldehydo and hydrazido groups. They allow in vivo hydrogel formation upon mixing.
Furthermore, functionalization of clickable polysaccharide strands with bioactive ligands such as cyclic RGD pentapeptides as cell adhesion factors has been achieved in an orthogonal manner. In that way libraries of polysaccharide-based hydrogels were created including those that are based on different polysaccharide backbones.
These studies are complemented with studies on their biophysical properties, biocompatibility and biodegradability as well as substitutes for extracellular matrices.
Biocompatible artifical lung
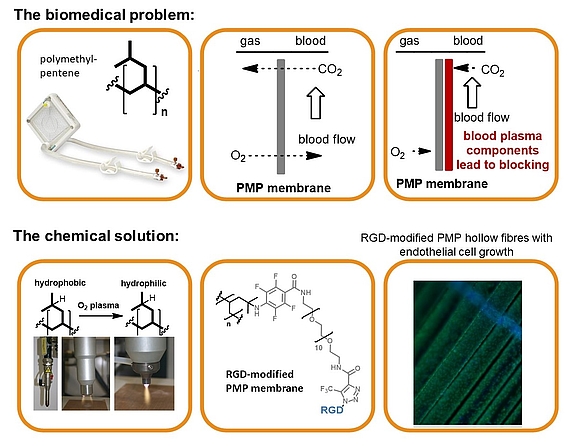

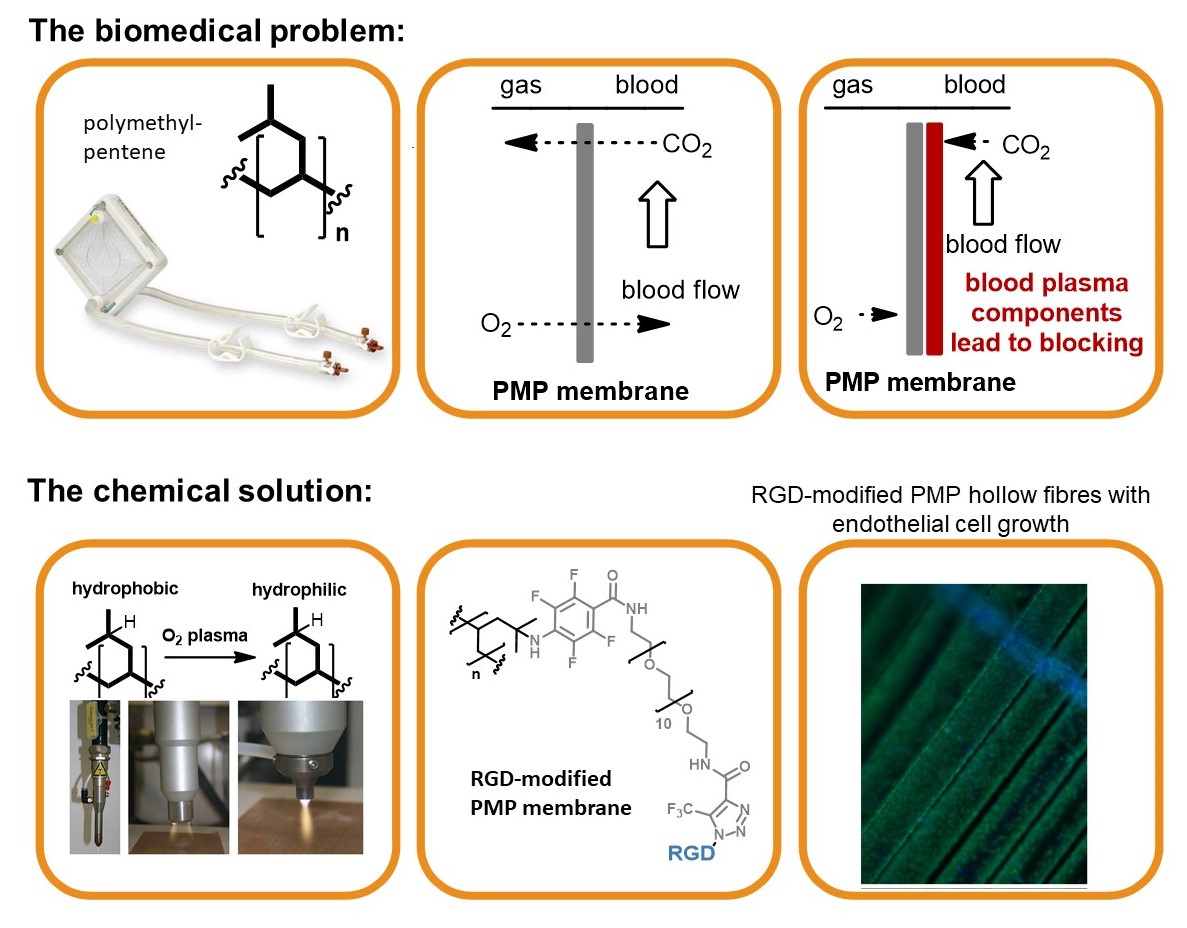
The biomedical problem: Respiratory failures are a significant health-care problem with several hundred thousand adult patients each year. The use of mechanical oxygenators (left) that provide breathing support while the recovery of the lungs occurs, is often indispensable. Extracorporeal devices are based on polymeric hollow fiber membranes (central), that serve as interface between blood and gas streams. In order to improve the biomedical properties and prevent blocking, endothelial cells (ECs) can be seeded onto the membrane surface (right). Nevertheless the cells show only low surface adhesion, which must be improved by attaching adhesion factors such as cyclic RGD peptide.
The chemical solution: Covalent multi-step coating of polymethylpentene (oxygen plasma, nitrene insertion, PEG coupling and copper-free click coupling of RGD) provided a highly biocompatible polymer surface. The resulting modified membrane preserves the required excellent gas flow properties while being densely seeded with endothelial cells.
SynBio: Biotransformations
Mutasynthesis: a synthetic tool in natural product chemistry
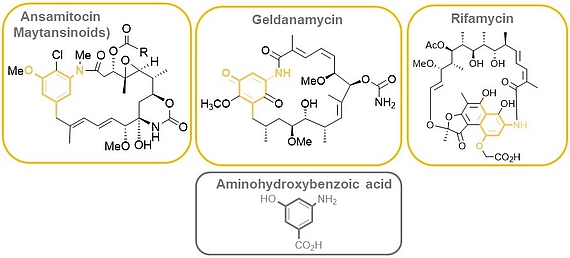
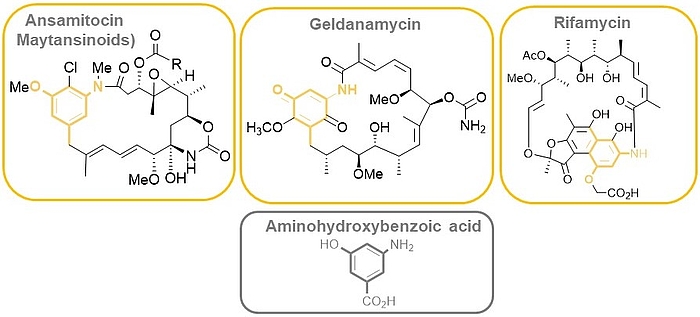
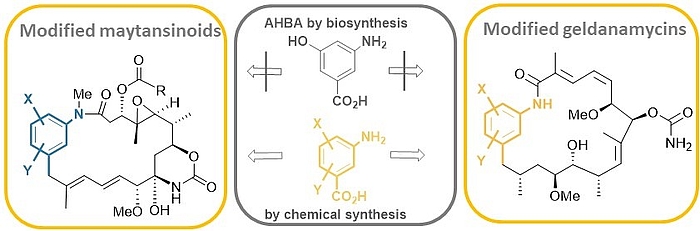
The ansamitocins (maytansinoids), geldanamycin as well as rifamycin are famous members of the class of ansamycin antibiotics which all bear aminohydroxybenzoic acid (AHBA) as polyketide synthase (PKS) starting building block.
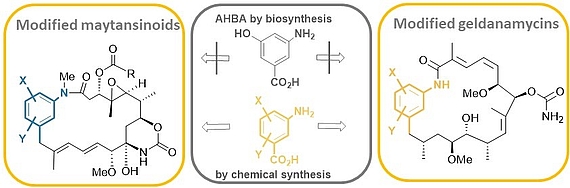
Mutasynthesis is a straightforward technique, that combines the genetic manipulation of biosynthetic pathways with chemical synthesis. It requires the generation of mutants of a producer organism blocked in the formation of a biosynthetic building block of the end-product. Administration of structurally modified, so called mutasynthons to these blocked mutant reconstitutes the substrate flow. However, in these cases the structural modifications result in new metabolites.
We carried out comprehensive mutasynthetic studies with three microbial strains (Actinosynnema pretiosum, Streptomyces hygroscopicus and Amycolatopsis mediterranei) blocked in the biosynthesis of aminohydroxybezoic acid (AHBA).
Promiscuity of terpene cyclases
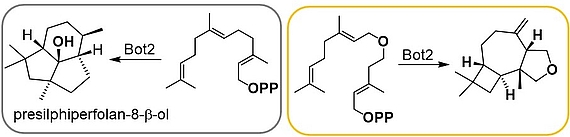
Terpenes are the most diverse class of secondary metabolites. They are generated from a linear precursor - in the case of sesquiterpenes this is farnesyl pyrophosphate (FPP) - by means of a cascade reaction. We demonstrated that sesquiterpene cyclases like presilphiperfolan-8-β-ol synthase (Bot2) show good substrate flexibility which allow to create new heteroatom-modified tricyclic sesquiterpenoids as shown for Bot2. For some new terpenoids GC-O analysis revealed an ethereal, peppery and camphor-like olfactoric scent.
SynMeth: methodology
Chemistry of hypervalent halides
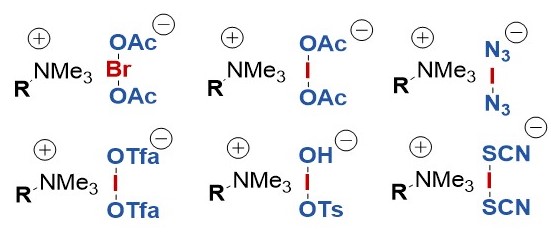


In the years 1998 and 1999 we reported on the preparation of a new class of electrophilic halonium reagents, which are obtained by iodine(III)-mediated oxidation of bromide and iodide. The resulting haloate(I)-complexes can be further diversified by ligand exchange. These reagents show a large synthetic versatility.
Oxidative decarbonylation of aldehydes
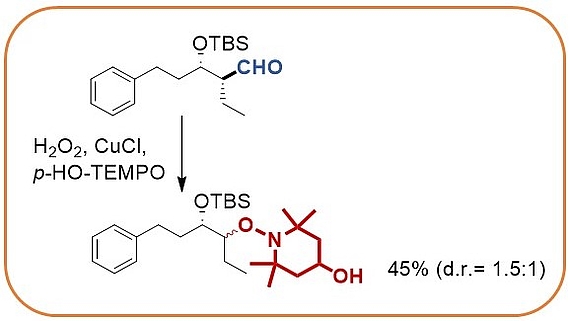
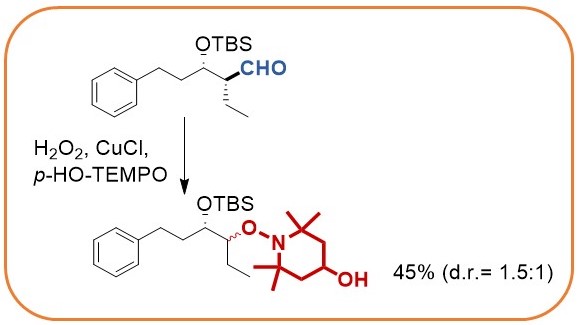

The stereocontrolled palladium catalyzed umpolung allylation utilizes diboranes to yield intermediate allylboranes that directly react with aldehydes. Stereocontrol is best achieved when chiral aldehydes are employed.
Asymmetric formylation of aldehydes



Our concept of the asymmetric formylation of aldehydes is based on dissecting the C-C bond forming process and the establishment of the newly formed stereogenic center. This is achieved by Horner-Wittig olefination of the aldehyde followed by Sharpless asymmetric dihydroxylation of the intermediate O,O-ketene acetal.
Flow: synthetic technology
Flow chemistry
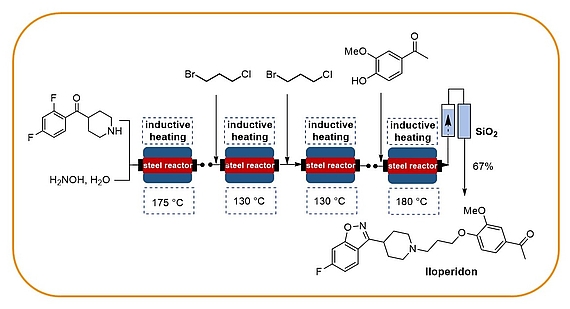
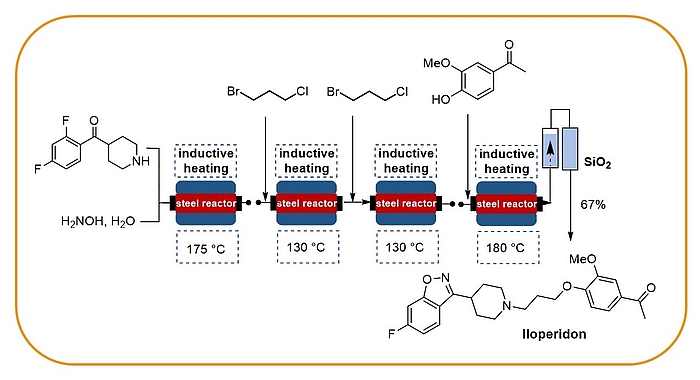
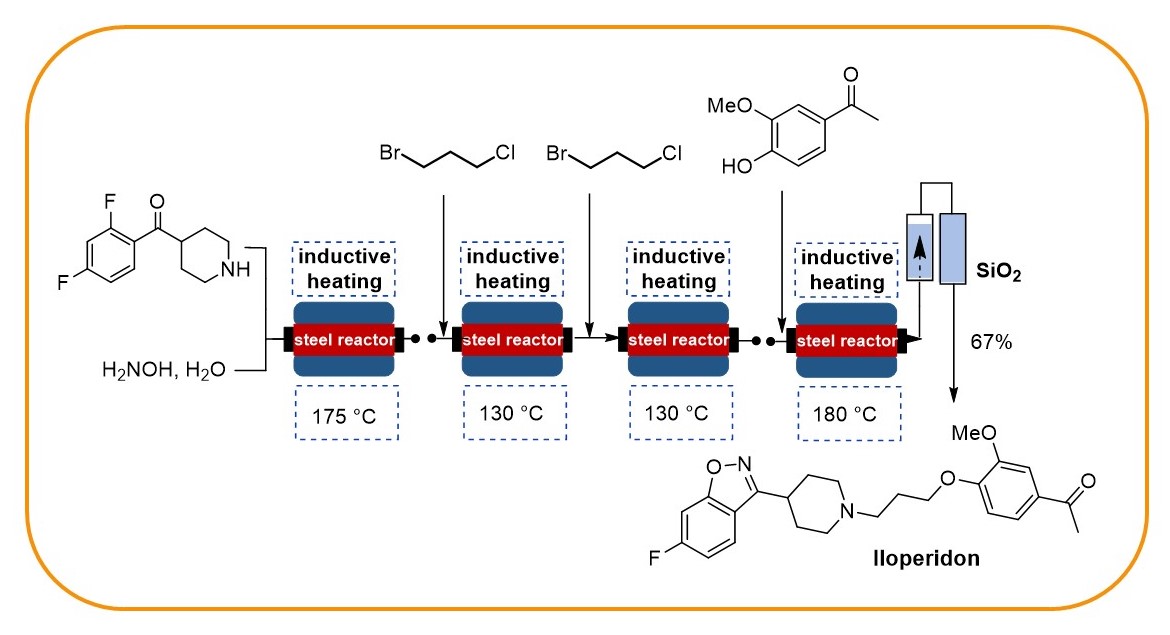
Our research programme on flow chemistry started in 1999 and led to a key publication in 2001 and an early key review in 2003. We cover the development of new, enabling technologies, immbolized (bio)catalysts, and multistep flow synthesis under continuous flow conditions. In 2008 we merged flow chemistry with inductive heating, an enabling technology suited to heat steel reactors or fixed-bed reactor materials based on copper or superparamagnetic nanoparticles (SPION). In combination with water as solvent this high temperature flow technology was exploited to carry out multistep syntheses of important drugs such as iloperidone.
Enabling technologies: inductive heating and flow
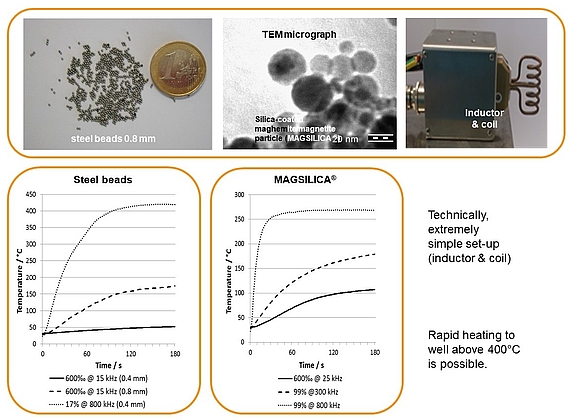
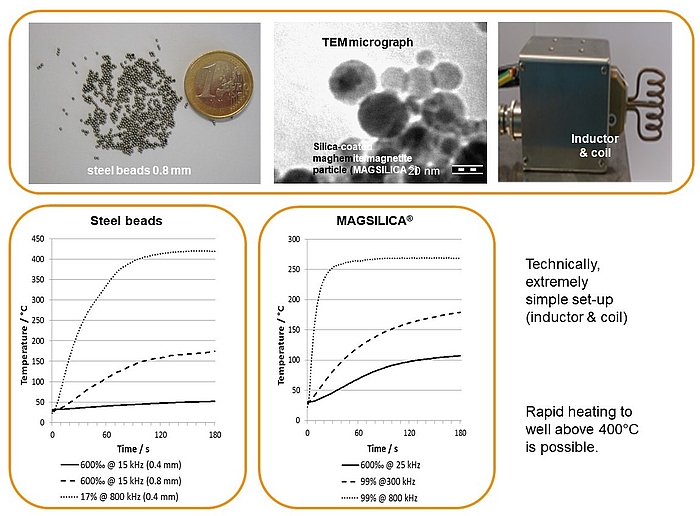
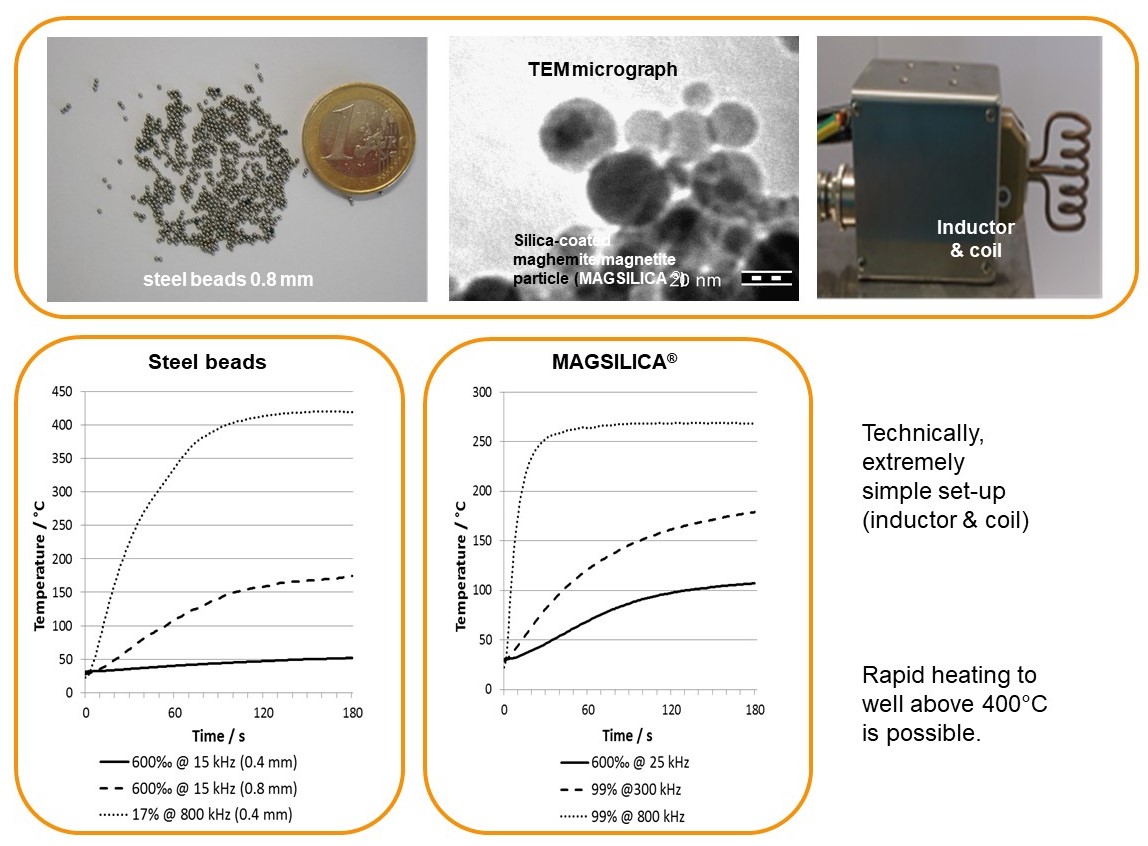
Inductive heating and mesofluidic reactors are a perfect match of two enabling technologies. Ferromagnetic materials (e.g. steel beads, copper metal) or superparamagnetic nanoparticles heat up in an oscillating electromagnetic field with medium (MF: 15-25 KHz) or high frequency (HF: 780-850 KHz) using appropriate inductors and coils.
TotalSyn: Totalsynthesis
Our total synthesis programme is embedded in a strategic partnership with the Helmholtz Center of Infection Deseases (Braunschweig/Saarbrücken). Here, our activities include the structure elucidation of new biologically highly potent secondary metabolites, their total and semisynthesis followed by their biological and biomedical profiling. Many of these natural products show either antiproliferative or antibacterial activities.
-
Carolacton
Carolacton was isolated from Sorangium cellulosum and shows biofilm growth inhibition 0.005 µg/mL. Planktonic cultures are insensitive to Carolacton
-
Elansolid A1 and A2
Elansolid A1 and A2 were isolated from gliding bacterium Chitinophaga. They show antibacterial activity (including Staphylococcus aureus and Micrococcus luteus).
-
Cystobactamid 861-2
Cystobactamid 861-2 was isolated from Cystobacter sp. and possesses strong antibacterial activity. This compound is a inhibitor of bacterial topoisomerase.
-
Thuggacin A
Thuggacin A was isolated from myxobacterium Sorangium cellulosum and shows strong antibiotic activity (including Mycobacterium tuberculosis). It targets the bacterial respiratory chain.
Targeted drug delivery
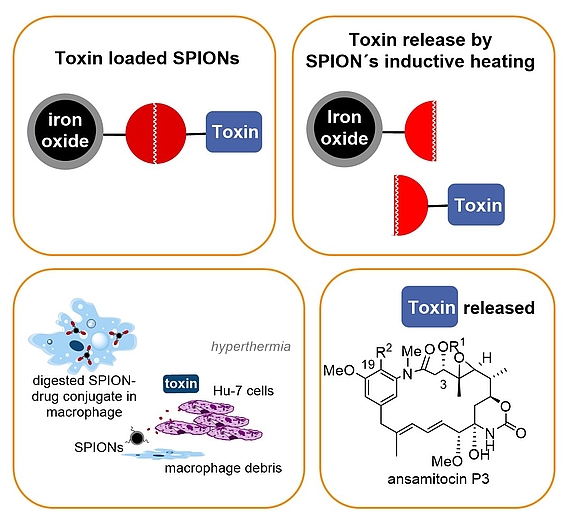
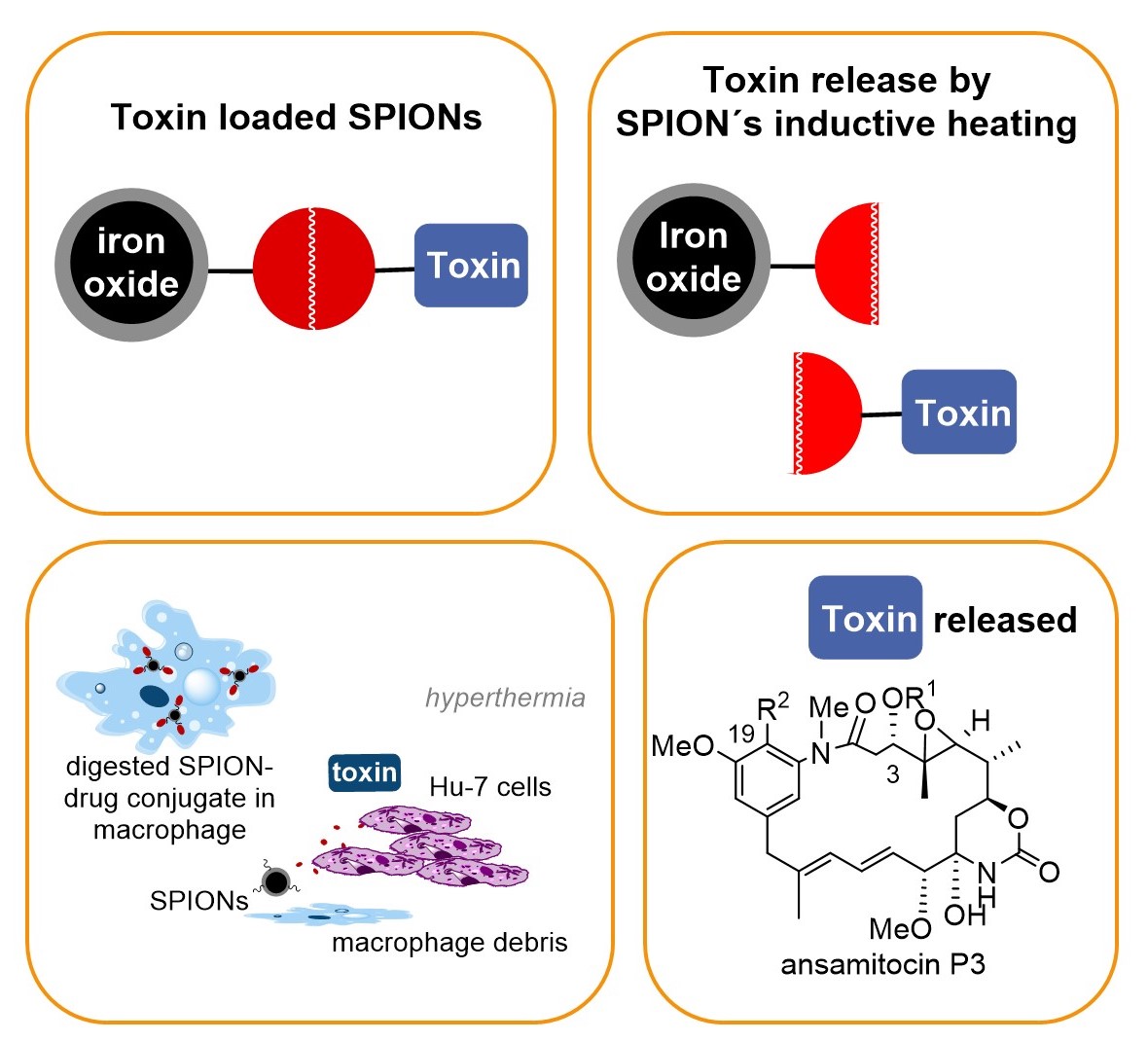
In our group a concept that combines drug delivery, targeting and release was developed that relies on nontoxic superparamagnetic ironoxide nanoparticles (SPION) – maytansinoid conjugates that bear a heat sensitive linker. Release of the toxic maytansinoid is achieved by inductive heating of the SPION which activates the thermosensitive linker. Proof of concept was demonstrated for cancer cell lines (in vitro) and for solid tumours (in vitro). For successful drug delivery and targeting we exploit the fact that in our case liver-specific macrophages are able to take-up nanoparticles including our SPION – maytansinoid conjugates and these macrophage transport systems undergo apoptosis followed by liberation of the toxin in an oscillating electromagnetic field.
Head of the group


30167 Hannover







